A solution of sodium sulphate in water is electrolysed using inert electrodes. The products at the cathode and anode are respectively:
(1) H2, O2
(2) O2, H2
(3) O2, Na
(4) O2, SO2
If a salt bridge is removed from the two half cells, the voltage:
(1) drops to zero
(2) does not change
(3) increases gradually
(4) increases rapidly
Two electrolytic cells, one containing acidified ferrous chloride and another acidified ferric chloride are connected in series. The ratio of iron deposited at cathodes in the two cells when electricity is passed through the cells will be:
(1) 3:1
(2) 2:1
(3) 1:1
(4) 3:2
Pick out the incorrect statement
(1) Equivalent conductance increases with dilution
(2) Molar conductance increases with dilution
(3) Specific conductance increases with dilution
(4) Specific resistance increases with dilution
The cell potential for this reaction is 0.46V. Which of the following change will increase the potential the most ?
(1) concentration is doubled
(2) [Cu2+] concentration is halved
(3) Size of Cu(s) electrode is doubled
(4) size of Ag(s) electrode is decreased to half
What will be the value of for the rection
if
(1) -65.62 KJ
(2) -75.27 KJ
(3) -150.54 KJ
(4) +150.54 KJ
The cell reaction of micro electrochemical cell fromed during rusting of iron is:-
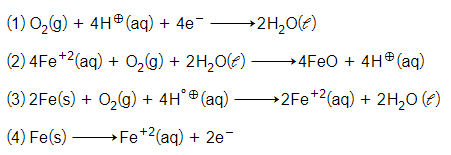
For the cell reaction
the change in free energy at a given temperature is a function of
(1) nC1
(2) n
(3) n (C1 +C2)
(4) nC2
The Emf of the following voltaic cell is:
\(\text {Ni }| \text {Ni}^{2+} (1 \text M) || \text {Au} ^{+3} \text {(1M) |Au}\)
\(\left ({~\mathrm{E}_{{\mathrm{Ni}^{+2}}/{\mathrm{Ni}}}^0=-0.25 \mathrm{~V}, \mathrm{E}_{{\mathrm{Au}^{+3}}/{\mathrm{Au}}}^0=1.50 \mathrm{~V}}\right )\)
1. 1.25 V
2. -1.75 V
3. 1.75 V
4. 4.0 V
The same amount of electric current is apassed through aqueous solution of MgSO4 and AlCl3. If 2.8 g Mg metal is deposited at amount of Al metal deposited in second cell will be
(1) 2.1 g
(2) 2.49 g
(3) 3.15 g
(4) 3.73g






