Two electrolytic cells, one containing acidified ferrous chloride and another acidified ferric chloride are connected in series. The ratio of iron deposited at cathodes in the two cells when electricity is passed through the cells will be:
(1) 3:1
(2) 2:1
(3) 1:1
(4) 3:2
Pick out the incorrect statement
(1) Equivalent conductance increases with dilution
(2) Molar conductance increases with dilution
(3) Specific conductance increases with dilution
(4) Specific resistance increases with dilution
The cell potential for this reaction is 0.46V. Which of the following change will increase the potential the most ?
(1) concentration is doubled
(2) [Cu2+] concentration is halved
(3) Size of Cu(s) electrode is doubled
(4) size of Ag(s) electrode is decreased to half
What will be the value of for the rection
if
(1) -65.62 KJ
(2) -75.27 KJ
(3) -150.54 KJ
(4) +150.54 KJ
The cell reaction of micro electrochemical cell fromed during rusting of iron is:-
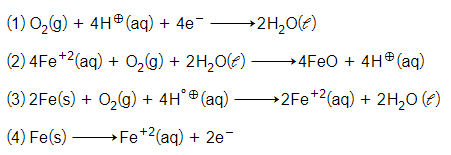
For the cell reaction
the change in free energy at a given temperature is a function of
(1) nC1
(2) n
(3) n (C1 +C2)
(4) nC2
The Emf of the following voltaic cell is:
1. 1.25 V
2. -1.75 V
3. 1.75 V
4. 4.0 V
The same amount of electric current is apassed through aqueous solution of MgSO4 and AlCl3. If 2.8 g Mg metal is deposited at amount of Al metal deposited in second cell will be
(1) 2.1 g
(2) 2.49 g
(3) 3.15 g
(4) 3.73g
A device that converts energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as
(1) fuel cell
(2) electrolytic cell
(3) dynamo
(4) Ni-Cd cell
gas can’t be obtained by the electrolysis of any salt because-
(A) Fluorine is the strongest reducing agent
(B) Fluorine is the strongest oxidising agent.
(C) Fluorine easily combine with atmospheric
(D) All






