Sulphide ores of metals are usually concentrated by froth flotation process. Which one of the following sulphide ores offers an exception and is concentrated by chemical leaching?
1. Argentite
2. Galena
3. Copper pyrite
4. Sphalerite

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Identify the incorrect statement
| 1. | The scientific and technological process used for isolation of the metal from its ore is known as metallurgy |
| 2. | Minerals are naturally occurring chemical substances in the earth's crust |
| 3. | Ores are minerals that may contain a metal |
| 4. | Gangue is an ore contaminated with undesired materials |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the conversion of Zinc ore to zinc metal, the process of roasting involves
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
The extraction of silver is achieved by the initial complexation of the ore (Argentine) with X followed by reduction with Y. X and Y respectively are
1. and Zn
2. and Cu
3. and Zn
4. and Zn

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Van Arkel method of purification of metals involves converting the metal to a
1. Volatile compound
2. Volatile unstable compound
3. Non-volatile stable compound
4. Non-volatile unstable compound

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
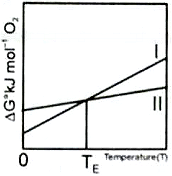
Which of the following is correct?
I. G for reaction (i) is more negative at T < 1125K
II. G for the reduction of CO is more negative at T < 1125K
III. is a better reducing agent at T < 1125K
IV. is a better reducing agent at T > 1125K
1. I and II
2. I and III
3. III only
4. I and IV

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Which of the following reactions is an example of auto reduction?
1.
2.
3.
4.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
A number of elements are available in earth’s crust but most abundant elements are ____________.
1. Al and Fe
2. Al and Cu
3. Fe and Cu
4. Cu and Ag

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
Zone refining is based on the principle that ___________.
| 1. | Impurities of low boiling metals can be separated by distillation. |
| 2. | Impurities are more soluble in molten metal than in solid metal. |
| 3. | Different components of a mixture are differently adsorbed on an adsorbent. |
| 4. | Vapors of the volatile compounds can be decomposed in pure metal. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.
In the metallurgy of aluminium ________________.
| 1. | Al3+ is oxidized to Al(s). |
| 2. | Graphite anode is oxidized to carbon monoxide and carbon dioxide. |
| 3. | Oxidation state of oxygen changes in the reaction at the anode. |
| 4. | Oxidation state of oxygen changes in the overall reaction involved in the process. |

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.

To unlock all the explanations of 20 chapters you need to be enrolled in MasterClass Course.






