The correct statement regarding ambident nucleophiles is:
1. Nucleophiles have equal atomic weight.
2. Nucleophiles have only five electrons.
3. Nucleophiles have two nucleophilic sites.
4. Nucleophiles have two electrophilic sites.
Any type of chromatography shares which of the following characteristic?
1. Use of molecules that are soluble in water.
2. Use of inert carrier gas.
3. Calculation of Rf value for the molecule separated.
4. Use of a mobile and a stationary phase.
The number of σ and π bonds in the molecule are -
| 1. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 2. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
| 3. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 4. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
The number of σ and π bonds in the molecule are:
1. 7 = ( , 11 = , and 0= π
2. 6 = ( , 12 = , and 0= π
3. 12 = ( , 6 = , and 1= π
4. 5 = ( , 13 = , and 1= π
Indicate the σ and π bonds in this molecule
1. two C–C sigma ( bonds, two C–H sigma bonds, and one C=C pi bonds
2. four C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
3. two C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
4. two C–C sigma ( bonds, two C–H sigma bonds, and two C=C pi bonds
The correct number of σ and π bonds in the molecule is:
| σ (C - H) | σ (C - N) | σ (N - O) | π (N=O) | |
| 1. | 1 | 1 | 1 | 3 |
| 2. | 1 | 1 | 3 | 1 |
| 3. | 1 | 3 | 1 | 3 |
| 4. | 3 | 1 | 1 | 1 |
The correct IUPAC name is -
| 1. | 2,2-Dimethylpentane | 2. | 2-Dimethylpentane |
| 3. | 1-Dimethylpentane | 4. | 2,2-Dimethylethane |
The correct IUPAC name of the following structure is

| 1. | 2,4,7-Trimethyloctane | 2. | 2,5,7-Trimethyloctane |
| 3. | 2,3,7-Trimethyloctane | 4. | All of the above |
The IUPAC name of the following molecule is -

| 1. | 2-Chloro-4-methylpentane | 2. | 4-Chloro-2-methylpentane |
| 3. | 4-Chloro-4-methylpentane | 4. | 2-Chloro-2-methylpentane |
The species present below is
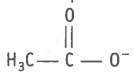
1. carbocation
2. electrophile
3. nucleophile
4. carboanion






