Regardless of the specific technique used (GC, HPLC, TLC, etc.), what is the fundamental mechanism required for all chromatographic separations to occur?
1. Use of molecules that are soluble in water.
2. Use of inert carrier gas.
3. Calculation of Rf value for the molecule separated.
4. Use of a mobile and a stationary phase.
The number of σ and π bonds in the molecule are -
| 1. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 2. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
| 3. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 4. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
The number of σ and π bonds in the molecule are:
1. 7 = ( , 11 = , and 0= π
2. 6 = ( , 12 = , and 0= π
3. 12 = ( , 6 = , and 1= π
4. 5 = ( , 13 = , and 1= π
Indicate the σ and π bonds in this molecule
1. two C–C sigma ( bonds, two C–H sigma bonds, and one C=C pi bonds
2. four C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
3. two C–C sigma ( bonds, four C–H sigma bonds, and two C=C pi bonds
4. two C–C sigma ( bonds, two C–H sigma bonds, and two C=C pi bonds
The correct number of σ and π bonds in the molecule is:
| σ (C - H) | σ (C - N) | σ (N - O) | π (N=O) | |
| 1. | 1 | 1 | 1 | 3 |
| 2. | 1 | 1 | 3 | 1 |
| 3. | 1 | 3 | 1 | 3 |
| 4. | 3 | 1 | 1 | 1 |
The correct IUPAC name is -
| 1. | 2,2-Dimethylpentane | 2. | 2-Dimethylpentane |
| 3. | 1-Dimethylpentane | 4. | 2,2-Dimethylethane |
The correct IUPAC name of the following structure is

| 1. | 2,4,7-Trimethyloctane | 2. | 2,5,7-Trimethyloctane |
| 3. | 2,3,7-Trimethyloctane | 4. | All of the above |
The IUPAC name of the following molecule is -

| 1. | 2-Chloro-4-methylpentane | 2. | 4-Chloro-2-methylpentane |
| 3. | 4-Chloro-4-methylpentane | 4. | 2-Chloro-2-methylpentane |
The species present below is
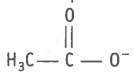
1. carbocation
2. electrophile
3. nucleophile
4. carboanion
ion is a-
1. carbocation
2. nucleophile
3. electrophile
4. carboanion






